-40%
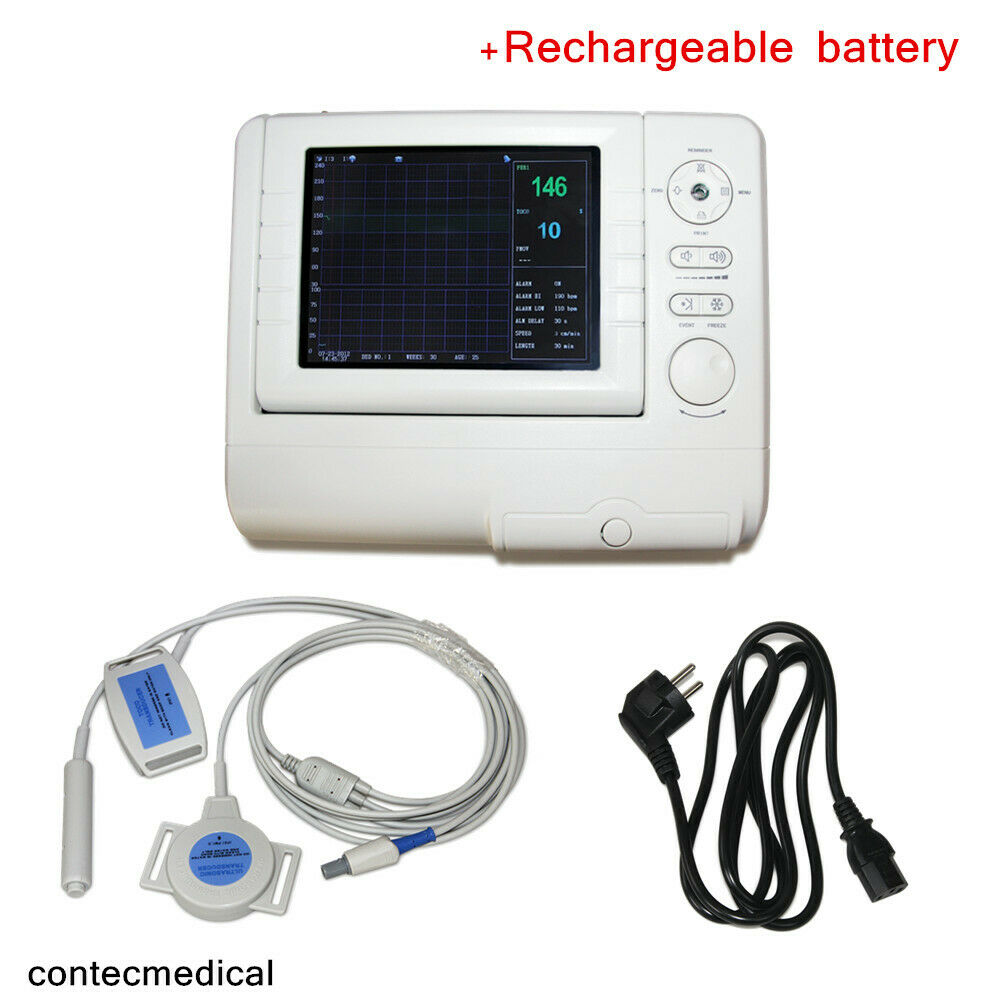
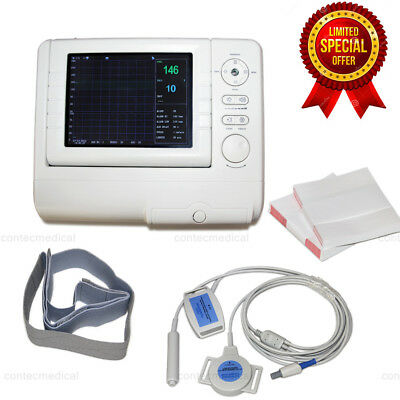
8.0"Fetal/Prenatal Heart Monitor,Ultrasound FHR TOCO Fetal Movement mark+battery
$ 242.35
- Description
- Size Guide
Description
8.0"Fetal/Prenatal Heart Monitor,Ultrasound FHR TOCO Fetal Movement mark+batteryInstructions
Fetal Monitor can real-time acquire FHR, TOCO and FMOV, which can provide reference data for clinical use. It can be used individually or connected with central monitoring system in obstetrical department to form a network monitoring system.
Function
1.Compact design, it can be placed horizontally or hung on the wall.
2.8.0" color LCD, 60° convertible screen
3.Display monitoring curve and data clearly
4.Area mark for normal FHR(120 BPM~160 BPM).
5.Manual record for fetal movement.
6.Alarm for abnormal FHR
7.Real-time monitoring for continuous 24 hours.
8.Storage, playback and print the monitoring curve for continuous 12 hours.
9.Optional interface languages: Chinese and English.
10.Optional twins monitoring.
11.9-crystal and broad beam transducer.
12.Extra long-life and high-resolution built-in thermal printer.
13.Communicate with central monitoring system by the built-in RJ45.
14.Built-in rechargeable lithium battery ensures that the device can work for 2 hours after unexpected interruption of power supply.
Specification
Safety
Type of protection against electric shock: Class Ⅰ
Degree of protection against electric shock: Type B applied part
Working Voltage:AC 100 V~240 V
DC 7.4V,3500mAh
Frequency: 50 Hz/60 Hz
P<60 VA
Fuse: T1.6AL250V
Battery type: rechargeable lithium battery, 3500 mAh/7.4 V
Display
8 "color LCD display, 60° convertible screen
Display Contents: bed No. , pregnancy weeks, age, paper speed, date, time, volume, reminder status, transducer connection status, recorder status, FHR data and wave, Contraction data and wave, Fetal move times and mark etc.
Record paper: Z-fold paper
Print width: 112 mm
Valid print width: 104 mm
Paper speed: 1 cm/min, 2 cm/min, 3 cm/min(optional)
Network interface: RJ 45
Ultrasound probe
Nominal Frequency: 1.0 MHz
Work Frequency: 1.0 MHz±10%
Negative peak sound pressure : p_<1 MPa
Output beam intensity : Iob<20 mW/cm2
The peak time space peak intensity: Ispta<100 mW/cm2
The average time space peak intensity: Isata<10 mW/cm2
FHR
Range: 50 BPM~240 BPM
Resolution: 1 BPM
Accuracy: ±2 BPM
TOCO
Range: 0~100%
Resolution: 1%
Nonlinear error: <±10%
RZ way: manual
Fetal Marking
Manual button
Accessories
A three-in-one Transducers(Ultrasound TransducerⅠ, TOCO Transducer, Remote Marker)
A User Manual
Two abdomen belts
An earth line
Record paper(two sets)
A power cord
Two Fuses
Physical Characteristics
Dimension: 320 mm (L) × 260 mm (W) × 80 mm(H)
Weight: about 3 Kg
Optional configuration
MPM1B21 Fetal monitor ultrasonic channel II probe(five core of raymer head)
Weight: about 3 Kg
Payment Way !!
* We accept payment from PAYPAL, Bank
transfer,Visa,Credit Card and others.
* All payments are expected within 7 days after the
last winning auction is closed.
Buy Safe Product!!
■The following FDA Disclaimer is required for all eBay listing in
Healthcare category and is included for REFERENCE:
■The sale of this item may be subject to regulation by the U.S.
Food and Drug Administration and state and local regulatory agencies.
■
If the item is subject to FDA regulation,We will verify your status
as an authorized purchaser of this item before shipping of the item.
■
The
model
is registered on the Australian Register of Therapeutic
Goods (ARTG)
with the code 197923,
and certified by FDA
510K Approved of America and CE,TUV of Europe
About Us
Contec Medical Systems Co., Ltd. (hereinafter referred to as CONTEC) focusing on research, manufacture and distribution of medical instruments, was founded in 1996 as a high-tech company. CONTEC locates in Economic & Technical Development Zone in Qinhuangdao covered an area of 125 acres and building area of over 100000 square meter, which is one of the largest bases for R & D and production of medical devices in China.
Contec hopes to cooperate with international companies to supply more innovative design and advanced
technology products
We sincerely welcome you to become one of our global partners.
We are looking forward to establishing a successful business relationship with you.
Powered by SoldEazy
Only English user guide,if you need any other language,please contact us!