-40%
CONTEC CMS800G Fetal Monitor Prenatal Heart FHR TOCO Fetal Movement Ultrasound
$ 242.35
- Description
- Size Guide
Description
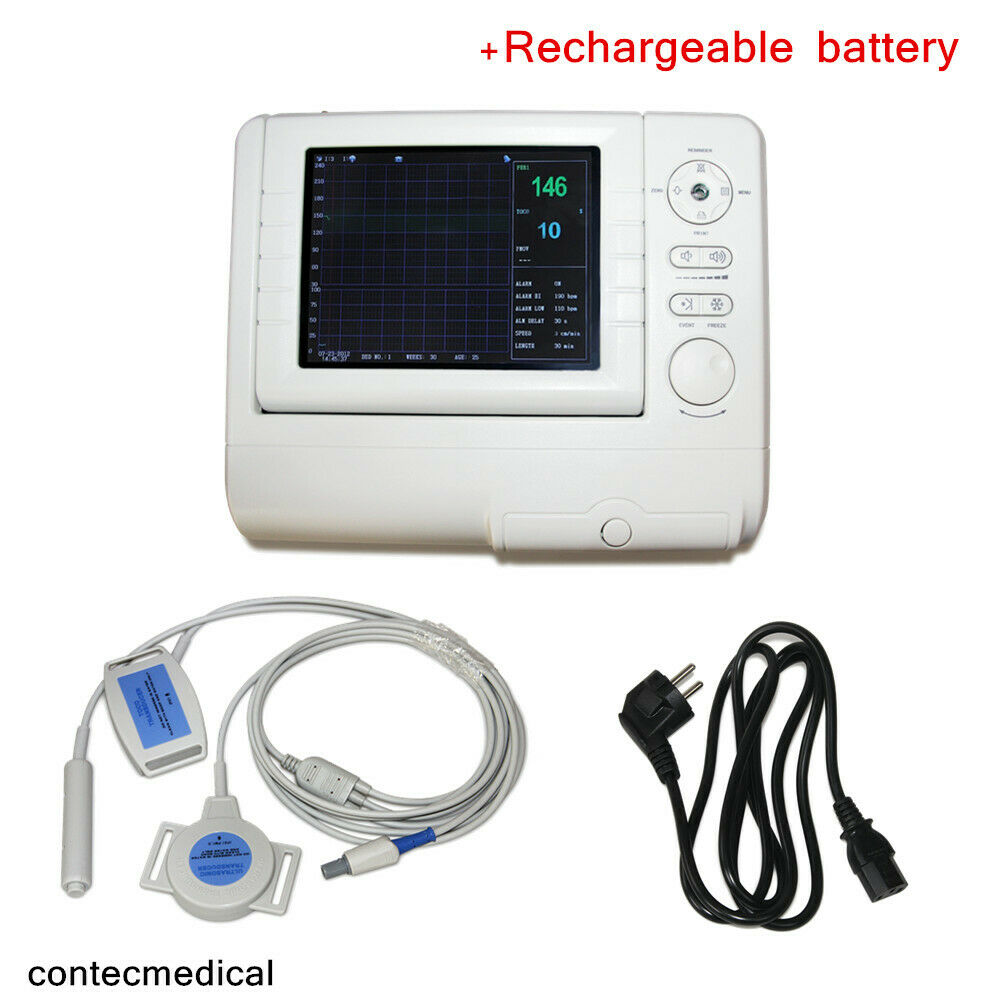
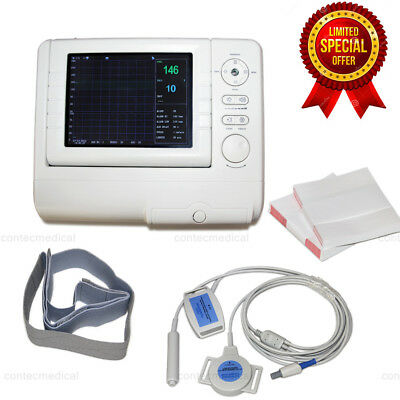
PM10CMS800G Fetal Monitor TOCO/FHR/Fetal Move
Description
Instructions
Fetal Monitor can acquire fetal heart rate, maternal uterine contraction when pregnancies over 28 weeks to provide reference data for clinical use.The monitor can be used individually or connected with PC through RJ45 Interface for the purpose of central monitoring.
It is only suitable for the equipment in hospitals, clinics, doctors offices and patients at home by professional medical personnel.
Major Features
◆Light dexterous appearance, tops horizontally and walls can be hoisted
◆8.0 "screen color LCD display, rotatable screen to 60°
◆Display of the patient data and curve clearly
◆FHR 120 BPM~160 BPM normal range label
◆Manual records fetal movement
◆Sound and color alarm for high and low fetal heart rate
◆Continuous 24-hour real-time monitoring function
◆Continuous 12-hour patient curve and data storage , playback and print
◆With picture freeze function
◆Optional English interface
◆Single, Twins Monitoring optional
◆9 crystal board band pulsed wave transducer
◆Extra-long life, high-resolution built-in thermal recorder
◆Built-in communication port, can be connected with central monitoring system.
Main performance
Security:
Anti-shock types: Facilities I, no internal power supply Anti-electric Shock Degree: B Working Voltage: AC 100 V~240 V Frequency: 50 Hz/60 Hz P<60 VA Fuse: T1.6AL250VDisplay
Dimensions: 8.0 "color LCD display, folding 60 degree
Display
Content: bed No. , pregnancy weeks, age, paper speed, date, time , volume, alarm status, transducer connection status, recorder status, FHR data and wave, Contraction data and wave, Fetal move times and mark etc.
Print:
Record Paper two-double type Z
Print Width: 112 mm
Valid Print Width: 104 mm
Paper output speed: 1 cm/min、2 cm/min、3 cm/min(optional)
Data Precision: ±5 %(X Roll),±1 %(Y Roll)
Record Content: hospital , bed No. ,name, pregnancy weeks, patient No., paper speed, date, time , FHR data and wave, Contraction data and wave, Fetal move times and mark etc.
Ultrasound probe:
Nominal Frequency: 1.0 MHz
Work Frequency: 1.0 MHz±10 %
Negative peak sound pressure : p_<1 MPa
Output beam intensity : Iob<20 mW/cm2
The peak time space peak intensity: Ispta<100 mW/cm2
The average time space peak intensity: Isata<10 mW/cm2
FHR Rang: 50 BPM~240 BPM
Resolution: 1 BPM
Accuracy : ±2 BPM
TOCO
TOCO range: 0~100 %
Resolution: 1 %
Nonlinear error: <±10 %
RZ way: Manually
Fetal Marking
For the manual button (the operation of pregnant women), there will be a mark display in the bottom area of FHR wave display section.
FHR Alarm:
Alarm for high and low FHR, which exceeds appointed limit.
Accessories
Sell in standard:
◆Transducers(Ultrasound TransducerⅠ, TOCO Transducer, Remote Marker)
◆Abdomen belt
◆Record Paper
◆Power supply line
◆Earth line
◆Two Fuses
◆User manual
Sell in addition
◆Ultrasound TransducerⅡ
Physical Characteristics
Size: 320 mm (length) × 260 mm (width) × 80 mm (height)
Weight: about 3 kg
Payment
1.Paypal
2.Bank transfer
Shipping
1. For your earlier and safer to receive the item, please leave us your detail address and telephone number.
2. Your item will be sent via Air mail within 48 hours after payment is received. The shipping time may is around 2-4 weeks.
3. As the usual way, buyer is responsible for the duties, taxes or other VAT or extra charges from the import countries.
4.All items will be shipped to buyer's PayPal address. Please confirm your PayPal address is correct before purchase.
International Buyers - Please Note:
? Import duties, taxes are not included in item price or shipping charges. These charges are buyer's responsibility.
? Please check with your country's customs office to determine what these additional cost will be prior to bidding
ying.
Terms of Sale
Buy Safe Product!
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved
Feedback
Please leave me a positive feedback if you are satisfied with our products, Your positive feedback will be appreciated,thank you.
If you have any problems, please contact us asap before you open a case and leave a negative feedback or ship the item back,
we promise we will give you a satisfied answer, thank you for your understanding!
Return - After service
We offer 60 days return policy. 100% satisfaction is our goal!
1.All items are brand new ,with 1 years warranty.
2.If you have a defective item, you want to return or discount. Please contact us within 2 days from you receive the item.
3.We will refund the money to you when we get the return items or replace item for you.
4. All return items must be returned with its original package and accessories. Customer is responsible for shipping charges on returned items.
About Us
Contec Medical Systems focusing on research, manufacture and distribution of medical instruments,was founded in 1992 as a high-tech company.
At present there are more than 1200 employees in our company.Our product line covers a wide range of 13 categories.Most of the domestic hospitals are our customers.Contec hopes to cooperate with international companies to supply more innovative design and advanced technology products.We sincerely welcome you to become one of our global partners.We are looking forward to establishing a successful business relationship with you.
Contact Us
Contact Person:Vivian Zhang
Contec Medical Systems Co.,Ltd, Manufacturer, Qinhuangdao, China
Address: No.112 Qinhuang West Avenue, Qinhuangdao Economic and Technical Development Zone, Hebei Province, China
Address(US):1440 Chase Avenue,Elk Grove Village,Illinois 60007 United States
Only English User Manual,Please contact us if you need other language manual.