-40%
Digital 3-Parameter FHR TOCO Fetal Movement Fetal Monitor Touch Screen +Paper US
$ 282.48
- Description
- Size Guide
Description
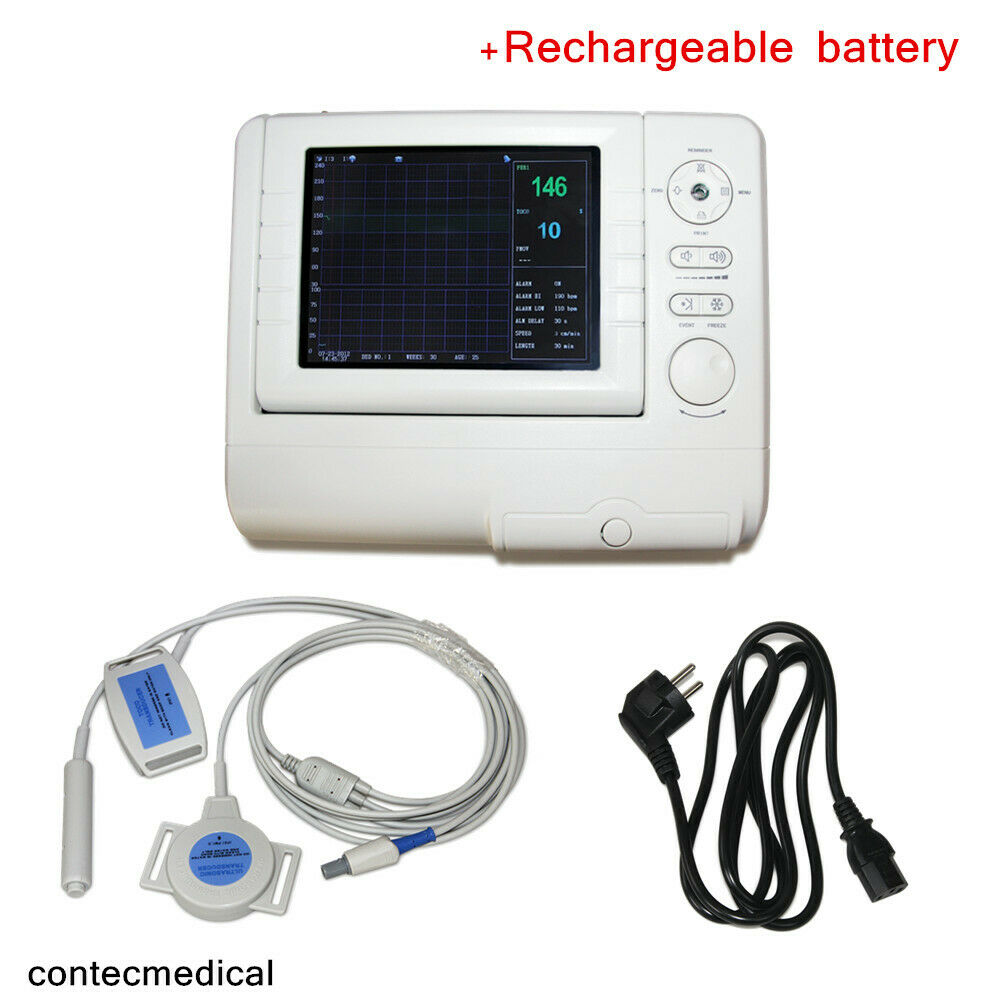
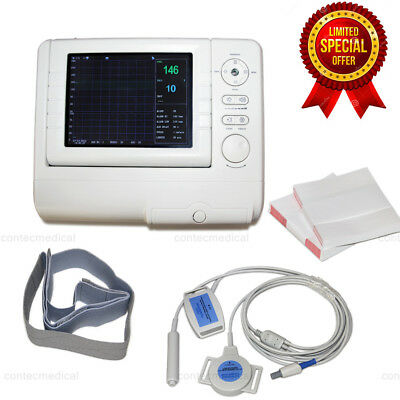
Product Overview7''Digital 3Parameters FHR TOCO Fetal Movement Fetal Monitor Touch Screen +Paper
Detail
7-inch LCD screen single montiroing Fetal Monitor with 3 Paramenters
Model: RFM-300B
Features
7" TFT Color display
Light weight, space-saving, easy to use
Display waveforms and digital simultaneously
24-hour real-time monitoring function
12 hours data and waveform storge review and print
FHR 120-160 bpm normal range label
Sound and alarm for high and low fetal heart rate
With picture freeze function
Single, Twins Monitoring optional
High sensitive 9 Elements 1 MHz pulse Doppler transducer
Built-in thermal recorder
Built-in wireless netnork, can be connected with central monitor system.
Parameters
Dimension of Recording
Paper: 110mmx20mm
Printing Speed: 1, 2, 3 cm/min.
Record Message: FHR trend graph, TOCO trend graph, time, paper, speed, etc.
Power Supply: AC: 100~240V, 50/60Hz
DC: 7.4V, 3700mAb
FHR
Technique: Ultrasound pulse doppler and auto correlation
Utrosound Frequency: 1 MHz
FHR Measuring Range: 0~100%
Resolution:1 bpm
Accuracy: ± 2 bpm
TOCO
TOCO Measuring Range: 0~100%
Resolution: 1%
Non-linear Error: ≤±10%
Zero Control: Manual
Fetal Movement
Dimension: 315(L)x215(W)x92(H)mm
Net Weight: 2.5 kg
Manual Fetal movement mark
Shipping Policy
Payment Mathord
Returns Policy
About Feedback
Contact US
Shipping Policy
1. We accept PAYPAL payment only and ship to your eBay address.
2. Item will be shipped within 1 business days after received a verified payment.
3. Shipped by air mail which normally takes 7-14 business days to USA, 12-25 business days to other countries.
4. Delivery Time upon purchase Locations
>>>Australia, South East Asia and Asia
15-18 working days
>>>United Kingdom, United States and Canada
8-21 working days
>>>Europe, South America, Middle-East Asia
16-28 wrokging days
>>>Italy and Brazil (because of strict custom inspection)
28-56 working days
Payment Mathord
1. PayPal payment accepted only.
2. Items will be shipped to your eBay address. Please make sure it is correct.
3. Payment must be received 4 days after auction ended.
Returns Policy
1. Customer satisfaction is our top goal. We believe our items are so outstanding. All products are quality checked. They are new and in good condition when shipped to our customers. We are convinced you will be happy with your Purchase.
2. If product is defective or damage upon arrival, or wrong product shipped, please contact us immediately. Returns accepted within 14 days of delivery date and item must be in original new condition, not worn or altered in any way with attached tags & wrap. Otherwise deal is final. Return shipping must be paid by buyer.
3. Please contact us first if you have any problems/questions/concerns. We will be happy to resolve any issues you may have in a cordial and friendly manner.
4. We appreciate your Postive Feedback, and will do the same in return. DO NOT leave negative feedback without first communication with us. Please allow max 2 business days for us to response.
About Feedback
When you satisfied with our product and services please leave us positive feedback.
If a problem occurs, contact us immediately with any email request. We promise we will give you the satisfied solution. :)
Contact US
The seller’s name: Alice
City:Beijing
State: Beijing
Country: China
Telephone number: 86-18305265146
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health: www.fda.gov/cdrh/devadvice/; www.fda.gov/cdrh/industry/support/index.html; Phone number: 800.638.2041;