-40%
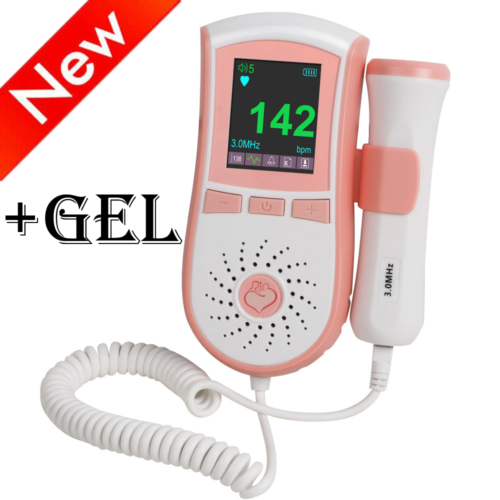
LED Backlight Fetal Doppler Prenatal Heart Fetus Monitor Dual Interface 3M Probe
$ 20.58
- Description
- Size Guide
Description
Description:Instructions:
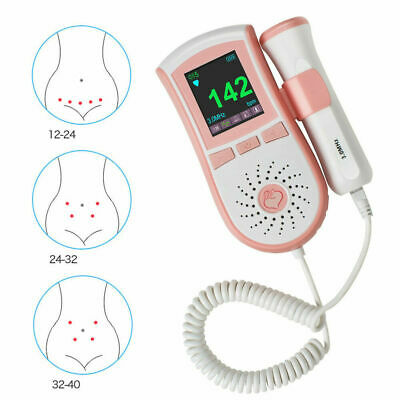
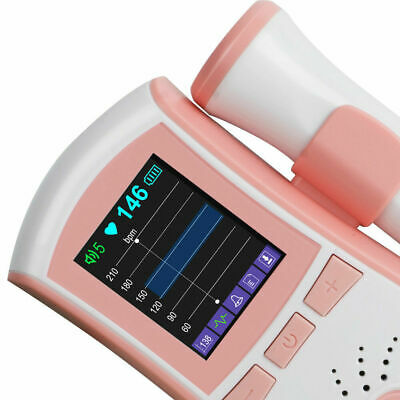
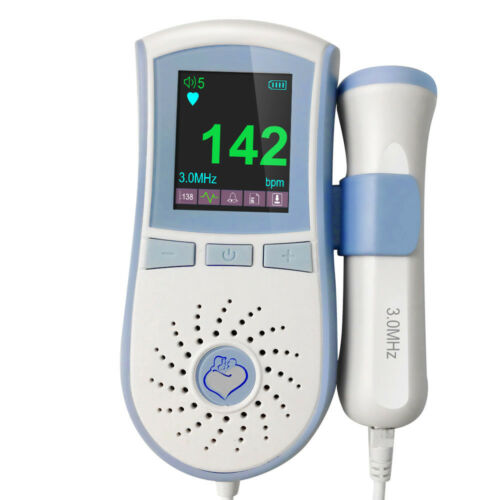
Pocket Fetal Doppler is a hand-held obstetrical unit, which can be used in hospital, clinic and home for daily self-check by pregnant woman.
The fetal doppler is equipped with a full high definition color LCD display.
Features:
Beautiful shape,portable,easy operation
Easy-to-use,the probe can be changed
Accurate FHR detection with clear sound
High sensitivity interchangeable probe
Battery status indicator
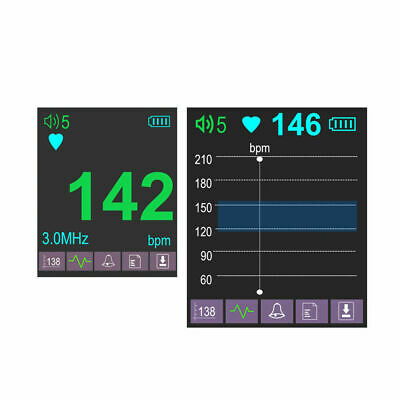
Dual
display Interface:Digital display interface, Curve display interface
10 set default user data storage function.
High accuracy, curve display interface, can see the fetal movement curve.
Specification:
Standard: EN61266:1995
Safety Classification: BF
/Class
Ⅱ
/Internal power
Overall Sensitivity:
≥
90 dB (Integrated sensitivity 200mm away from the surface of the probe)
Measuring integrated sensitivity
The Doppler frequency :(300 ± 50) Hz
Reflecting target speed:10cm / s ~40cm / s
Ultrasound transducer’s effective area: 6.0±0.5cm2.
Target velocity and display range : No narrower than 50bpm-240bpm (± 2bpm). (Beat Per Minute)
(Notes: Please go to see a doctor once the FHR is abnormal) Output power:
≤
10Mw/cm2
EMC testing data (See annex
Ⅱ
)
Working frequency:3.0 MHz
±
10%
Spatial-peak temp-peak acoustic pressure:
≤
0.1 MPa. Effective area of transducer:6.0
±
0.5 cm2
Physical Characteristic:
Working environment: Temperature: +5
℃
~ +40
℃
Humidity:
≤
80% Atmospheric pressure: 86kPa ~ 106kPa
Transport and storage environment:
Atmospheric pressure: 86kPa ~ 106kPa,well-ventilated room without corrosive gases.
Battery: Two pieces of 1.5V battery
Size: 135mm (Length) ×65mm (Width) ×30 (Height) mm
Weight: 176g(without batteries)
Probe:
Working Frequency:3.0MHz
P-:
≤
1MPa
Iob
≤
20mW/cm2
Ispta
≤
100mW/cm2
Packing Include:
1 x Color LCD Fetal Doppler
1 x English user's manual
Warranty
EVERY PRODUCT GOT 12 MONTH WARRANTY!!
FDA Disclaimer
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and stateand local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
This item has been cleaned and treated according to the manufacturer's instructions.(Seller Name: Mona Li;Country: China;City: Beijing;Phone No.:86-13971580721)
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.